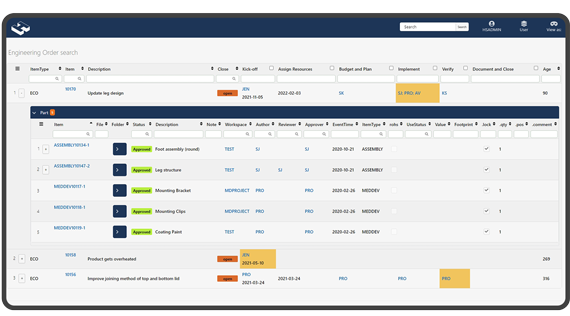
Customizable digital management solution.
Replace the traditional systems with a customizable digital management solution that simply documents and accelerates the pace of your work during development within the medical device industry.
Get in touchLet's improve your efficiency
Customizable processes
Compared to competitors, Highstage is priced making even small companies able to utilize the benefit of managing their development in a professional and structured way.
Fast implementation
Fast and easy implementation allowing you to speed up the process within 4-6 weeks.
Audit ready
Regulatory ISO/audits are welcome at any time, as normally no 'clearn up' is necessary
Control & simplicity
Every solution is designed to provide full overview for the managers and create simple workflows for the staff.
Medical device companies partnering with Highstage

Compatible with FDA CFR Part 11.
Ensure that all documents are managed by version/revision, and are signed for authoring, review and approval electronically according to FDA CFR Part 11 without tampering.

Easy management of technical files
Organize your technical files using the revision-locked references in Highstage. This guarantees that tech files are always intact and correct.
Use our powerful export features to pack and send entire technical files.

Version-controlled forms
Define all your checklists and forms as Highstage templates. This will make your checklists and forms version controlled and provides full traceability to the template source of an e.g. checklist.
It will also save you time every time you need to create a new document.

Full Audit Track
All changes to data are stored, and a full audit-track event log is accessible for provisioned stakeholders.

Safe and structured product release.
The Highstage PART module streamline processes and ensures that your new product can only be released when the whole assembly is approved.
Provides excellent status overview.

Automated document content
Documents in Highstage can be partly or completely automatically generated. Any metadata in the system can be automatically written into MS Office documents. This includes lists of referenced documents or parts, making it super fast and 100% correct to generate e.g. index lists for DMR's og BOM's.

Classify documents according to your standards
Implement the classification standards that you already use - being it international standards like STED or DIN, or your internal classification nomenclature.
This will give you simple searches, easy grouping of documents, etc..

Easy integration of tech and BOM files.
Manage your complete product in Highstage. Integrate generation of technical files with engineering and store tham in one large logical structure. Version locked references provide 100% control of technical documents from a BOM component and vice versa.

Instant status overview
The way Highsage colour-code documents and other information give your management a superior overview of the status on documentation, engineering and other processes. In addition, deadlines can be applied to any document or step.

Pre-validated
All main releases of Highstage are pre-validated by us. Customers have full access to download validation protocols, validation records and objective evidence in the form om video footage.
Configurations
The various Highstage modules can be used in a variety of combinations, depending on which business processes you want to improve. From the simplest document control system, to the corporate wide, full fledged, PLM System
Get demo or want to talk?
Get further with a customizable solution for medical devices. Fill out the form and we will get in touch.

"The argument that you don't have the time to implement Highstage and understand the values that form the platform, is something that needs to be challenged. It is without a doubt the best management system on the market"
Interim CEO
6 HOWs On why Highstage works for you
Highstage offers a solution that literally helps you at any stage of your work with medical device compliance. Based on smart digital modules that seamlessly fit together, we focus on your needs for future-proof agility.
Read more- Compliance is made faster through a completely new level of usability.
- Tech files are automatically generated and make work simpler and more flawless
- Automatic reports compile and reduce the work maintaining DHF/DMR indexes to a minimum
- Safe signatures are standard and can for medical device applications even be forced to adhere to FDA CFR Part 11
- Traceability is essential at all times; thus, all operations are recorded in the eventlog
- Document mapping lets you gain full control over the project by monitoring the status of the documents in the document map
Resources
You are more than welcome to contact our main office. But if more convenient, please contact one of these local partners.













